Cannabis: Progress in Peruvian Regulation
During the last years, the use of medical and therapeutic Cannabis and its byproducts has taken on a lead role thanks to researches on its health benefits. Peru is not unfamiliar with this progress and, in 2017, the first regulation on Cannabis for medical and therapeutic use (Act No. 30681) has been passed, amending the criminal code to decriminalize its use for such purposes.
Pursuant to this regulation, Supreme Executive Order No. 005-2019-SA, enacted in February 2019, it is set forth to establish a regulatory system of research, import, trading and production of Cannabis and its byproducts for medical and therapeutic purposes.
Peru is an attractive place for domestic and foreign investors due to its climate and geographical conditions favorable to sowing and cultivation of Cannabis.
Likewise, legalization of medical Cannabis is being implemented progressively in many American countries to enable patients to access to Cannabis in different types of preparations, either for relief symptoms, treat certain diseases or improve their quality of life.
In America, Cannabis legalization index is led by Canada and United States in the first and second place, respectively. Latin American countries having a Cannabis market potential are Uruguay, Peru, Colombia, Chile, and Mexico, among others.
Below, the implementation progress of Act No. 30681 in different entities related thereto are shown as follows:
1. DIGEMID – Requests for Cannabis sanitary/health registration
In 2019, the General Medicine, Input and Drug Administration (DIGEMID) accepted 9 requests for Cannabis products Sanitary/Health Registration from 5 companies having Health Authorizations for Operating as Drug Wholesalers/Distributors. Only one product has been submitted as a pharmaceutical product under category 2 in relation to High Health Standard Control Countries (Sativex)[1].
To date, no Sanitary/Health Registration has been issued.
2. DIGEMID – Registry of Cannabis Patients
In 2019, the registry of Cannabis patients has been kept active and with growing tendency. As of October 30, 1277 patients are registered as per public information requested by DIGEMID through online Request for Access to Public Information (SAIP) No. 19-008910.
Submitting patients to the National Registry of Patients Using Medical and Therapeutic Cannabis is online at the link below enabled on DIGEMID website.
Click here.
3. DIGEMID – Drug Wholesalers/Distributors and Laboratories
DIGEMID updated the A-2 and A-LPF forms regarding Health Authorizations for Operating as drug wholesalers/distributors and laboratories, specifying the medical Cannabis byproducts businesses: herbal medicine, pharmaceutical product, health natural product.
(http://www.digemid.minsa.gob.pe/Main.asp?Seccion=624).
Currently, drug wholesalers/distributors with medical Cannabis byproducts businesses have been authorized. To date, there is one request for Health Authorizations for Operating as laboratory in order to perform business in medical cannabis and byproducts that is under evaluation.
4. Peruvian Police Force Anti-Drug Division (DIRANDRO) – Security Protocols
Security protocols aim to secure physical intangibility of medical and therapeutic Cannabis and its byproducts avoiding robbery, theft and other incidents generating quantitative loss of Cannabis and its byproducts for illegal purposes.
The security protocol for Cannabis import and distribution license is under an automatic evaluation and subject to a later control by the respective agency of the Ministry of Internal Affairs (MININTER), as per Section 16.1 of the Regulation of Cannabis Act (Supreme Executive Order No. 005-2019-SA).
In this sense, the security protocol having the pertinent seal affixed granted by DIRANDRO reception desk constitutes the acknowledgement and proof of reception. Security protocols approval requirements and terms vary according to the requested type of license.
To date, there are many companies that have been submitted their security protocols to DIRANDRO.
5. DIGEMID – Import and Production Licenses
Drug Wholesalers/Distributors conducting trading business of medical Cannabis byproducts can request the Cannabis import and trading license to DIGEMID.
Steps and requirements are as follows:
6. SENASA – phytosanitary requirements pending to be published
The National Service for Agricultural Health (SENASA) set forth public consultations of projects pursuant to Director’s Resolution for establishing mandatory phytosanitary requirements for import of Cannabis seeds (Cannabis sativa) of origin and provenance from Colombia and United States[2].
Deadlines of both public consultations were April 20 and May 31, respectively.
They are still pending to be published.
| PROPOSED REGULATION | START OF CONSULTATION | END OF CONSULTATION |
| Proyecto de Resolución Directoral para el establecimiento de requisitos fitosanitarios de necesario cumplimiento en la importación de semillas de Cannabis (Cannabis sativa) origen y procedencia Colombia
(Bill of Director’s Resolution to establish mandatory phytosanitary requirements for import of Cannabis seeds (Cannabis sativa) of origin and provenance from Colombia) |
2019/03/20 | 2019/04/20 |
| Proyecto de Resolución Directoral para el establecimiento de requisitos fitosanitarios de necesario cumplimiento en la importación de plantas de semillas de cannabis (Cannabis sativa) de origen y procedencia Estados Unidos
(Bill of Director’s Resolution to establish mandatory phytosanitary requirements for import of Cannabis seeds (Cannabis sativa) of origin and provenance from United States) |
2019/05/02 | 2019/05/31 |
7. SENASA classifies Cannabis extracts under phytosanitary risk 1
SENASA confirmed that the hemp oil obtained by carbon dioxide extraction and dried Cannabis flowers extract heat- and chemically- treated is a product under phytosanitary risk 1. Therefore, it is not subject to mandatory control by SENASA within import process.
SENASA responses to consultations are at our clients’ disposal.
8. INS – Technological newsletter on Medical Cannabis
The Peruvian Health Institute (INS) through its General Office of Research and Technological Transfer prepared a newsletter in order to set a baseline on research and development for Cannabis and its byproducts for medical and therapeutic purposes exclusively as per Act No. 30681.
As of publication of such newsletter—May 2019—11 patent requests were being processed.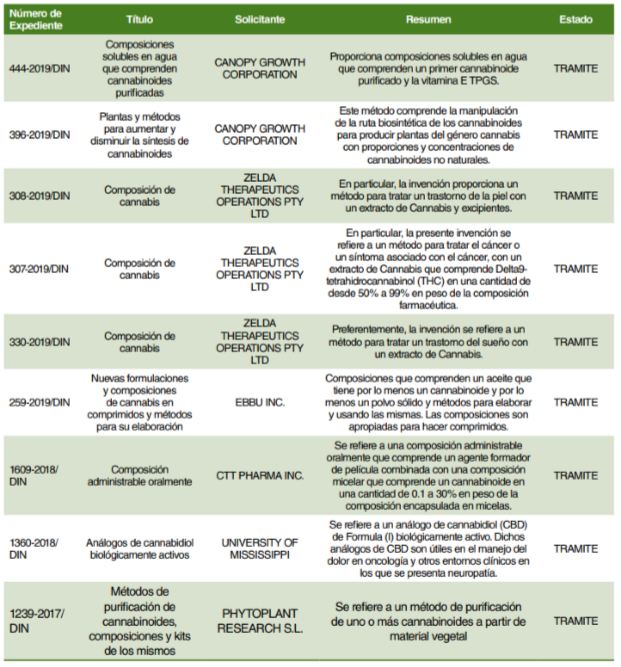
For newsletter, access here.
9. INS – Presenting health research projects
INS has developed a health research project record system named PRISA that can be accessed at the following link.
This website shows information of health researches conducted by entities and individuals in Peru devoted to research.
To date, a “Pilot study on cannabidiol effect on the behavior associated to depression by reserpine in adult rats”.
For more information about said study, click here.
For further details, please contact Maritza Reátegui (mreategui@estudiorodrigo.com), Solange Noriega (snoriega@estudiorodrigo.com) and/or Cecilia Alarcón (calarcon@estudiorodrigo.com).